
The Pulmogine® Vibrating Mesh Nebulizer delivers different types of medication with an easy-to-use and effective system.

The Pulmogine® Vibrating Mesh Nebulizer delivers different types of medication with an easy-to-use and effective system.
Simple and intuitive
One bottom design
IP55 protection
Water resistant
Light-weight
Hand-held size
Higher delivery rate
Shorter nebulization time
Sound <50 dB
Quiet treatment

The Pulmogine® Vibrating Mesh Nebulizer incorporates the leading-edge Micro Synchronized Delivery TechnologyTM
High performance
Deliver medicine to lower airways
Recommended by pulmonary specialists and pediatricians
Pulmogine has obtained regulatory approval in the United States, European Union, Australia, Taiwan, and China.
>5μm
Upper Airways
nose, mouth, sinuses, pharynx, and larynx
1-5 μm
Lower Airways
trachea (windpipe), bronchial tubes, and lungs
<1μm
Alveoli
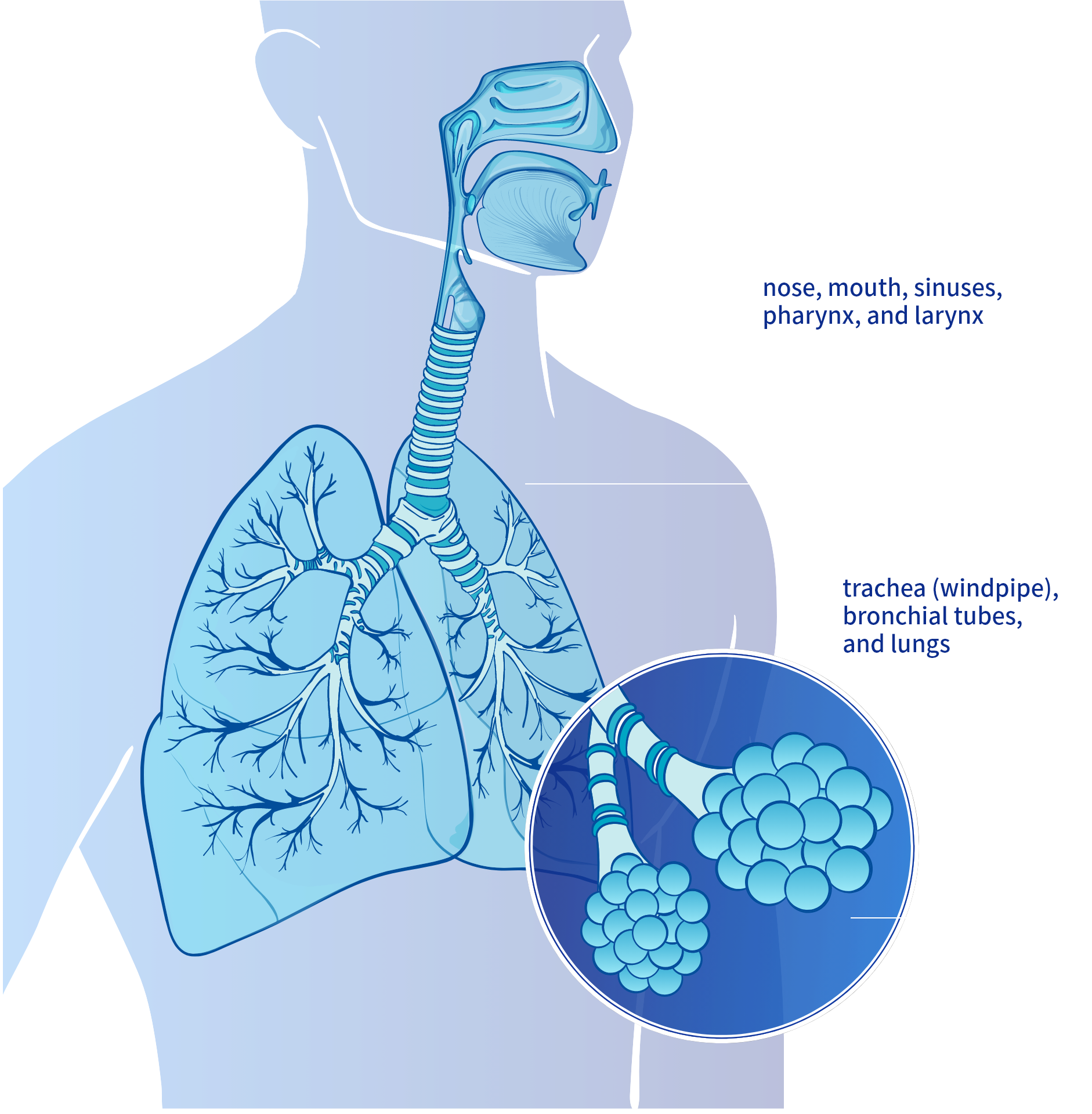
The palladium alloy mesh membrane of the Pulmogine® Vibrating Mesh Nebulizer generates fine particle aerosol below 5µm in diameter to reach lower airways.
The Pulmogine® Vibrating Mesh Nebulizer can be used to nebulize a wide range of liquid medications to treat respiratory diseases and their symptoms in children and adults.
Asthma
Bronchitis
Cystic Fibrosis
Chronic Obstructive Pulmonary Disease
Common cold symptoms
Non-Cystic Fibrosis Bronchiectasis