Nebulize High Value Therapeutic Drugs
Scroll down
HCmed 堅持三個核心價值,作為我們行動的指導原則。
-
熱情
-
信任
-
效率
全球先進吸入藥物遞送平台的合作夥伴,
專門提供
呼吸偵測
以患者為中心的
智慧型裝置
客製化
創新的
解決方案。
我們的產品

連續式霧化裝置

呼吸偵測式霧化裝置
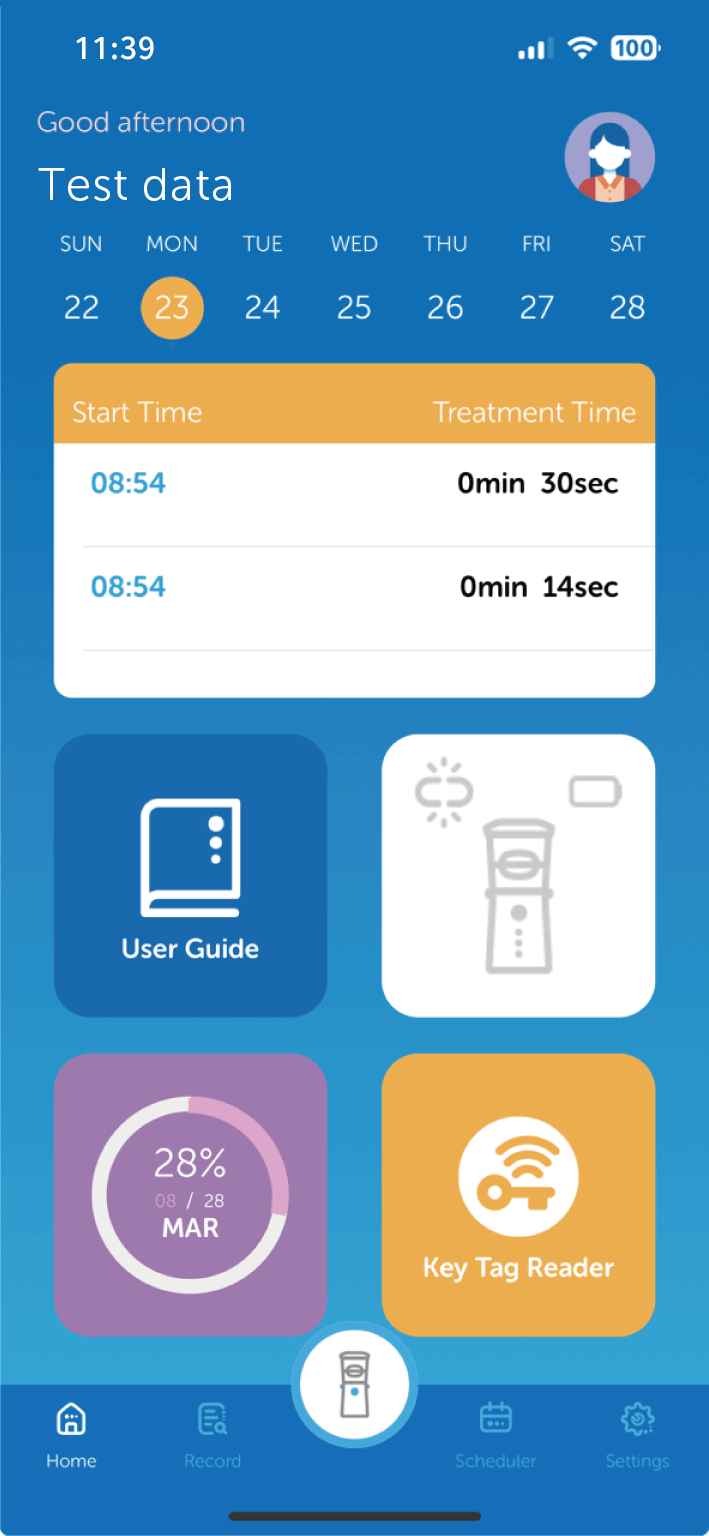
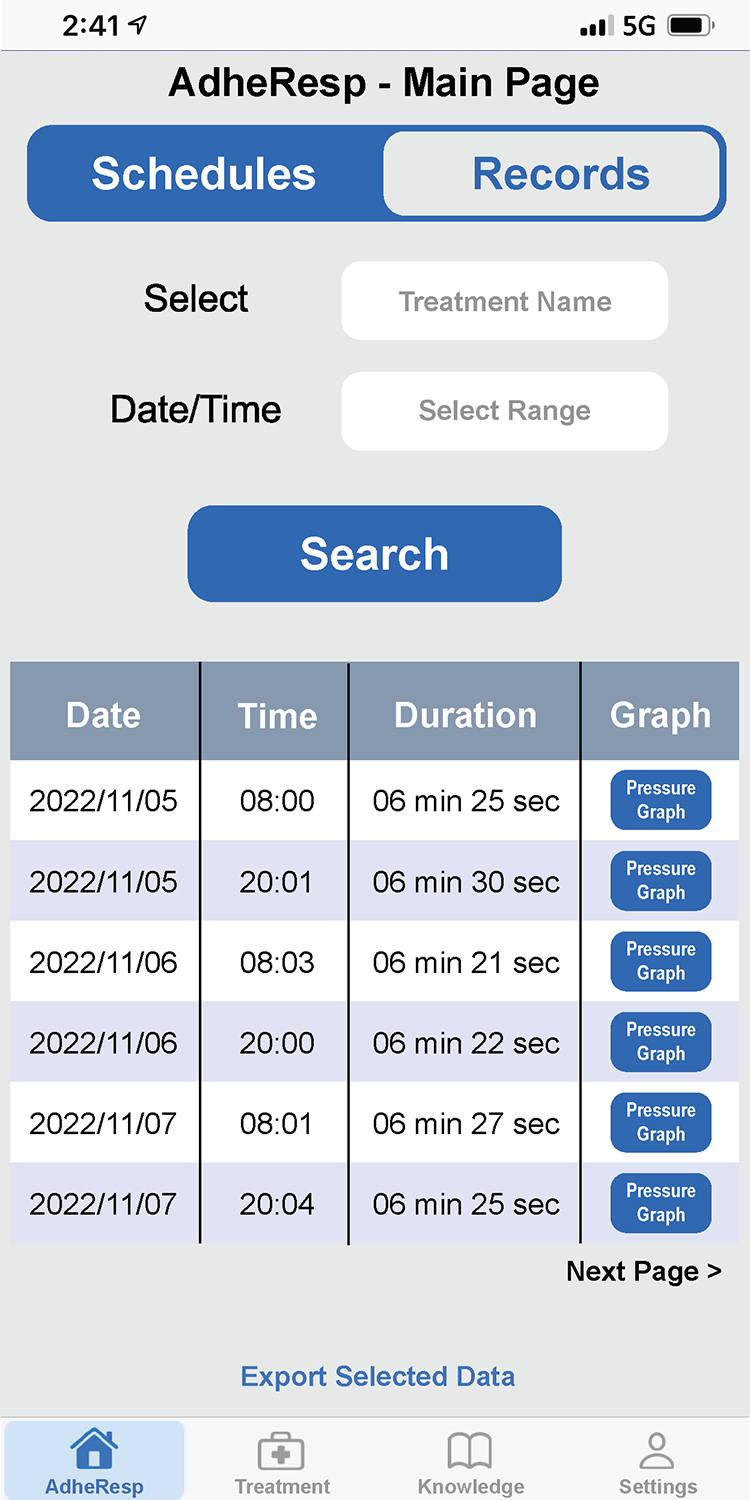
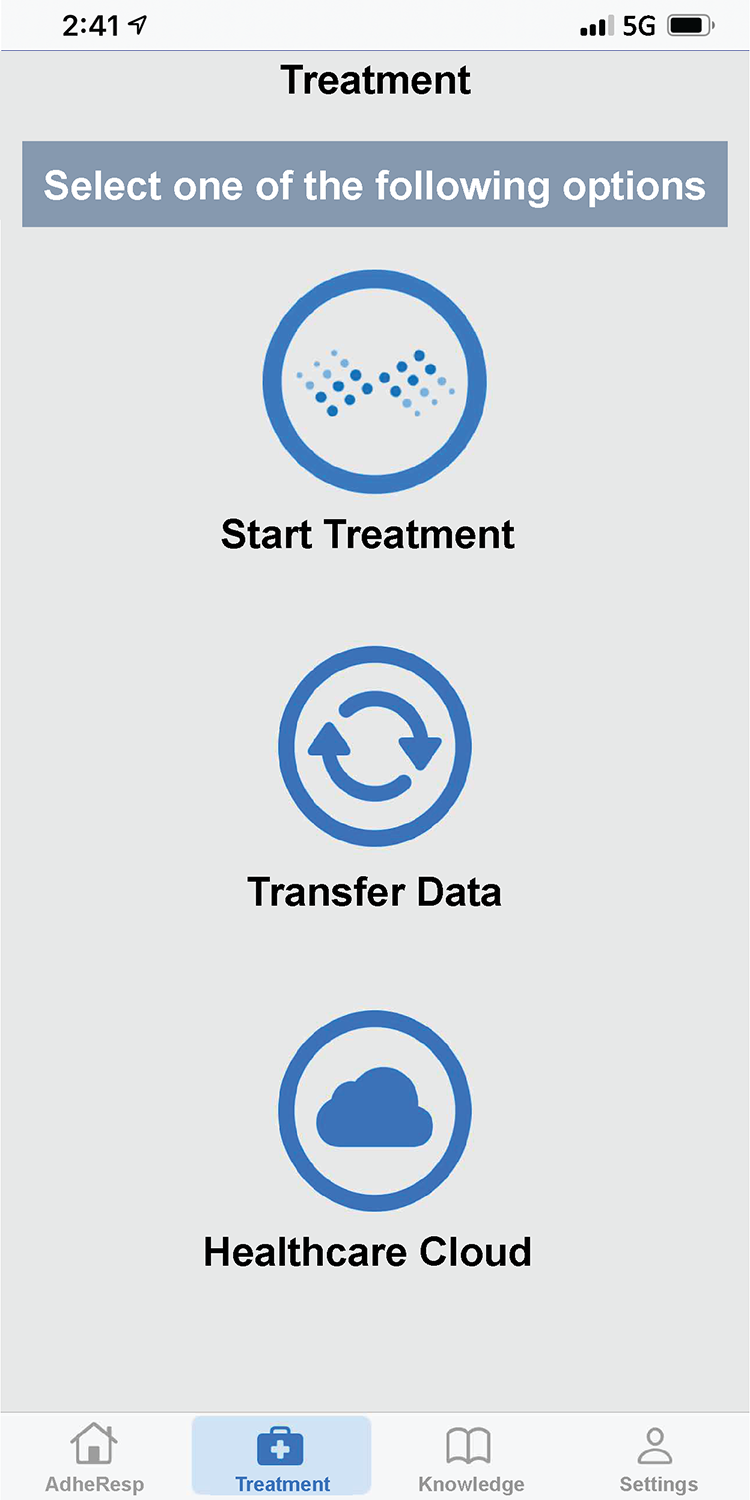
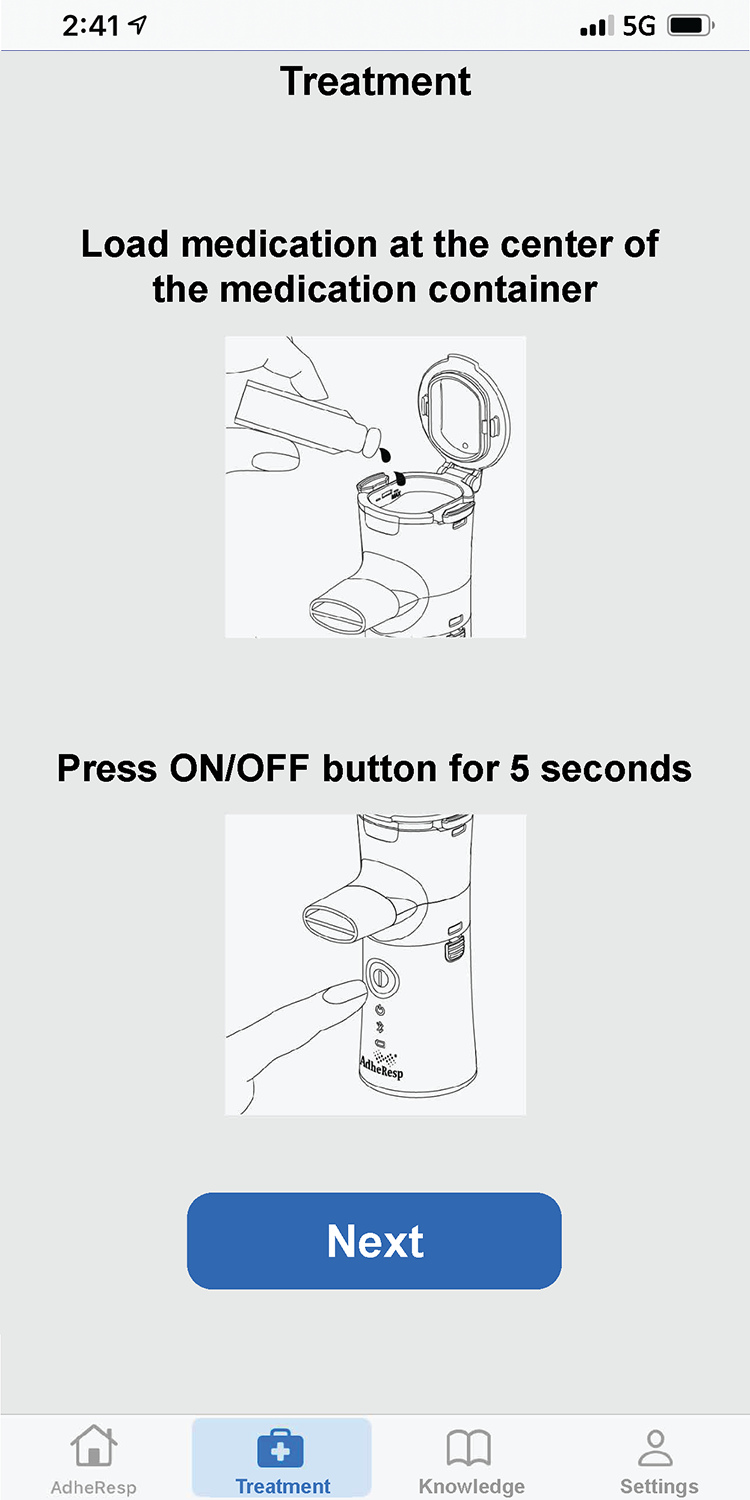
AdheResp行動應用程式
AdheResp呼吸偵測式霧化裝置的行動應用程式,能透過手機與裝置連線,記錄病患治療狀況及用藥之依從性(adherence)。
此應用程式利用藍芽連線功能來傳輸和儲存治療數據,並同步至雲端資料庫。此應用程式包含治療紀錄、流程指示、提醒功能及裝置使用教學資料等.
*僅供展示用

技術與服務
憑藉多年研發經驗,心誠鎂擁有業界領先的先進技術,從概念至商品化,我們提供一站式的吸入性藥械合一客製化開發平台。
-
呼吸偵測啟動
-
藍芽連線
-
客製化開發平台
-
臨床試驗支援
-
文件支援及量產
-
商品化